Chemical Sourcing in Drug Discovery and Controlled Substances
A controlled substance is generally a compound that has its manufacture, acquisition, possession and use regulated by a government. In drug screening, controlled compounds may be extremely difficult, if not impossible, to acquire. Moreover, the storage, handling, and disposal of controlled compounds can be further challenging still. Therefore, identifying controlled compounds early-on in the process, and removing them from the compound library list can help streamline chemical sourcing.
However, identifying controlled compounds is not a straightforward task. Historically, purchasers would only find out about controlled compounds from the supplier after placing their order. This can result in unexpected cancellations, and delays to the order and delivery process. Ultimately, this is not just frustrating, but could lead to significant setbacks in the drug development project pipeline and in getting therapeutics to patients.
In this blog, we discuss the challenges associated with chemical sourcing for controlled compounds, including the difficulties associated with both complex chemical nomenclature and legislation. We then look at how innovative solutions that provide upfront and transparent information on controlled compounds can help streamline the chemical procurement workflow in drug discovery.
Is the compound controlled?
Drug libraries for screening can consist of thousands to millions of different compounds. For these large chemical libraries, simply identifying which compounds are subject to regulation is a massive and time-consuming task.
Adding to this challenge is the difficulty of searching legislation using keywords or chemical names. The variety of naming standards for chemical entities can make searching a time-consuming and complex task. Moreover, modern legislation tends not to regulate a single substance but employs “generic statements” in an attempt to control areas of chemical space that are likely to have similar properties.
For instance, 3,4-Methylenedioxymethamphetamine (MDMA), commonly known as ecstasy, is a controlled substance in the UK. However, it is not directly named in UK legislation. Instead, in Part I of Schedule 2, paragraph 1(c) of the Misuse of Drugs Act, MDMA is regulated under a generic rule for compounds structurally derived from Phenethylamine: “Any compound (not being methoxyphenamine or a compound for the time being specified in sub-paragraph (a)) structurally derived from phenethylamine, an N-alkylphenethylamine, αmethylphenethylamine, an N-alkyl-αmethylphenethylamine, α-ethylphenethylamine, or an N-alkyl-αethylphenethylamine by substitution in the ring to any extent with alkyl, alkoxy, alkylenedioxy or halide substituents, whether or not further substituted in the ring by one or more other univalent substituents.”
An additional challenge is the variation in legislation between different countries (Table 1). Therefore, to purchase a controlled substance, you would need to consider the country of origin, the final delivery location, and also any country that the shipment may pass through (e.g. the country of your 3rd party reformatting partner).
Table 1. Examples of generic legislation regulating controlled substances in different jurisdictions.
| Jurisdication | Regulation |
| Belgiuim | BE arrêté royal réglementant les substances stupéfiantes, psychotropes et soporifiques |
| UK | UK Misuse of Drugs Regulations 2001 Schedule I |
| UK | UK Third Generation Synthetic Cannabinoids |
| UK | UK Misuse of Drugs Act Part I Class A Drugs |
| Ireland | IE MISUSE OF DRUGS REULATIONS 1988 Schedule I |
| Switzerland | CH Swiss Controlled Substances Act (BetmVV-EDI) Narcotics List E |
| Singapore | SG Misuse of Drugs Act FIRST SCHEDULE Controlled Drugs |
| Italy | IT Decreto del Presidente della Repubblica del 9 ottobre 1990, n. 309 TABELLA I |
| Denmark | Dk Bekendtgørelse om euforiserende stoffer Liste b |
While molecules such as codeine are controlled across most jurisdictions worldwide, others are only controlled in a few areas. Caffeine, for example, is tightly controlled by China’s National Medical Products Administration (NMPA), but is largely unregulated and legal in nearly all other parts of the world.
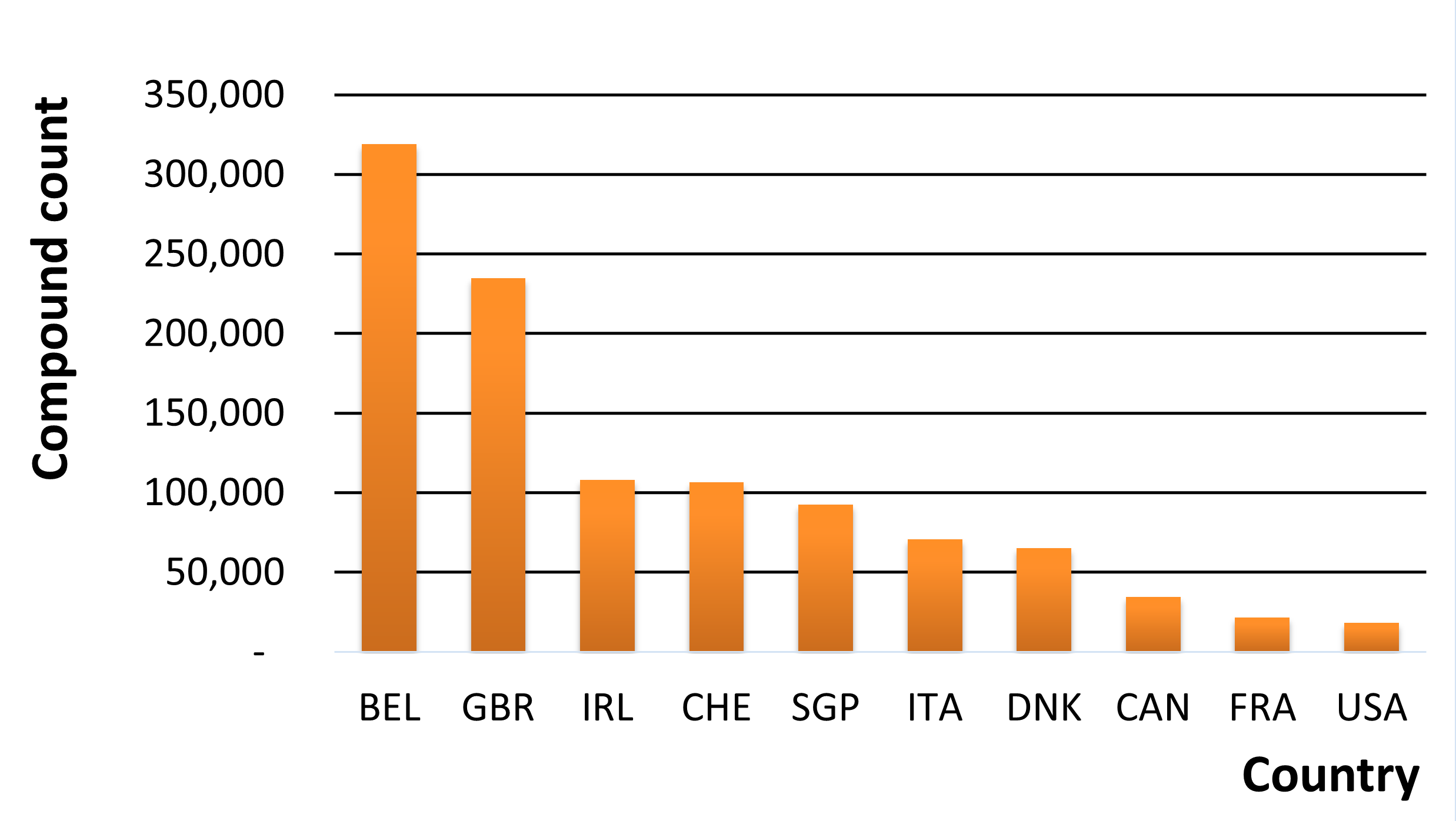
From a broader perspective, we can visualize the differences in legislation by looking at the number of controlled compounds in each country in MolPort’s database (Fig.1). MolPort’s database holds a total of 483,960 compounds that are controlled in any one or more countries. Belgium has the largest number of controlled compounds (318,912) – more than 17-fold more than the number controlled in the United States (18,352).
Having access to clear, transparent, and easily searchable information at an early stage is therefore critical to successful identification and procurement of controlled compounds.
How to handle controlled substances?
In some cases, it is possible to acquire a controlled substance specifically for drug screening. However, this involves navigating the complex landscape of regulation compliance. This means sorting through a lot of documentation, acquiring the correct import/export licenses, and continuously monitoring legislation to ensure ongoing compliance.
Additionally, if your screening efforts highlight a controlled compound as a potential drug candidate, its use in downstream steps may still be subject to different regulations. In other words, you might need to go through the effort of complying with a new set of regulations if you want to continue using a controlled compound downstream.
As a result, in nearly all situations, it is simpler to just remove all controlled substances from your compound library list.
Controlling the uncontrollable
Removing every controlled substance from your compound library still requires identifying them. So, what is the answer?
The solution is to have clear, upfront, and transparent data regarding the regulatory status of your entire compound library list, prior to placing an order. For example, MolPort’s open access database has recently integrated data from ChemAxon’s Compliance Checker; a tool designed to screen chemical structures efficiently against controlling legislation.
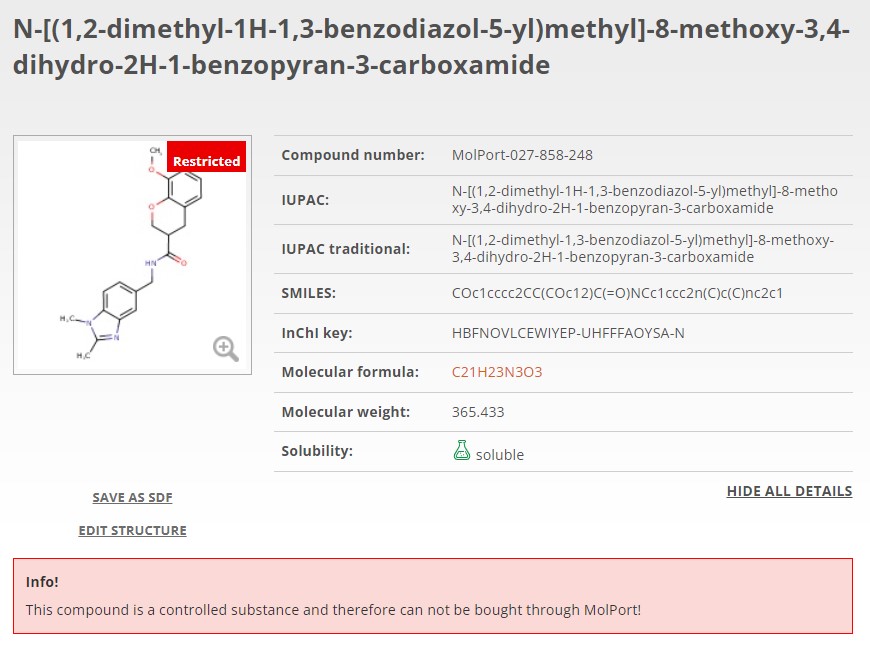
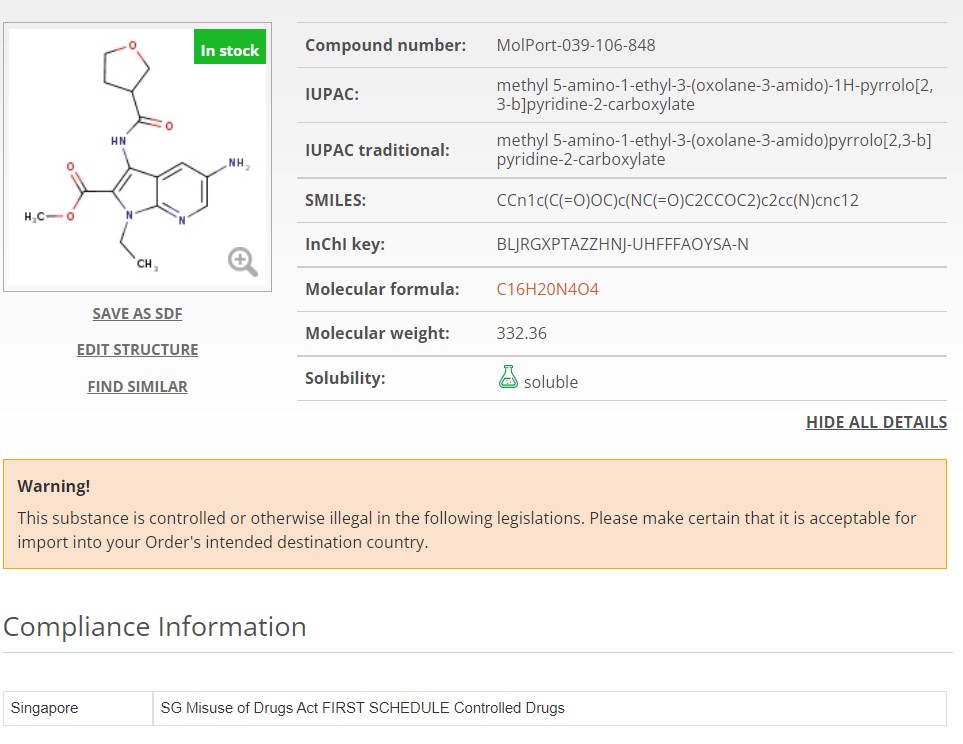
Now, when you search for a molecule or list of molecules, any controlled substance will instantly be flagged or excluded. In MolPort’s case, compounds known to be controlled in at least two countries have been removed from the databases (FTP, API, online) in anticipation of evolving regulations elsewhere. This simplifies logistics and minimizes the risk of selecting compounds that might become controlled in the future (Fig. 1A). If a compound is controlled in only one country, it is still available for purchase however, it will be clearly flagged on the molecule page and again if added to the shopping cart. Additionally, accompanying regulation information including the country of regulation and its legislation is clearly displayed (Fig. 1B).
Transparent, upfront, and open-source information like this can help scientists streamline chemical sourcing, and is at the heart of what MolPort does. From regulatory information to stereochemistry, pricing and availability, MolPort’s intelligent compound platform provides free and clear information on the attributes important to chemical sourcing workflows.